SERVICES AND TESTS AVAILABLE
○ We provide a variety of genomic tumour-based tests and Liquid Biopsy
○ We conduct hereditary genetic testing and counselling
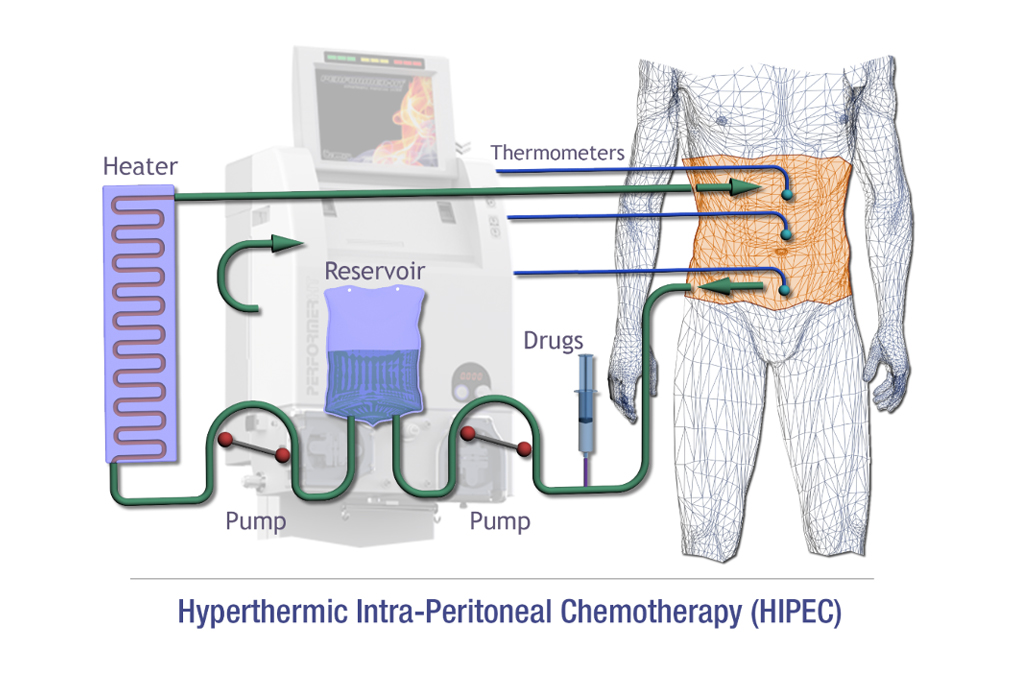
○ We support intraoperative chemotherapy include HIPEC, Isolated limb perfusion
○ We provide individualised assessment of cancer recurrence risk based on genomic markers including OncotypeDx for breast cancer, and HalioDx Immunescore for colon cancers
○ We perform autologous and allogeneic haematopoietic cell transplantation for acute leukaemias, multiple myeloma and other indications
○ We perform apheresis for haematopoietic stem cell harvest and plasmapheresis for immunological conditions
Diagnostic Tests
Accurate diagnosis of tumours is the foundation of all cancer therapy. Understanding a tumour’s precise molecular alterations will allow for very focused targeting of tumour using “targeted”…
TEMPUS is a test that is able to perform DNA, RNA and whole exome sequencing of a patient’s tumor It may potentially provide actionable…
PARP inhibitors have been labelled as one of the biggest breakthough for medicine and oncology. myChoice CDx tumour testing, allows for the precise selection of patients likely to benefit…
Lucence tests support cancer diagnosis, treatment selection, and disease monitoring for improved cancer care. Through identifying…
FoundationOne CDx is a FDA-approved broad companion diagnostic (CDx) that is clinically and analytically validated for solid tumors. The test provides next-generation DNA sequencing…
This medical grade blood test evaluates up to 84 genes associated with hereditary cancers including breast and gynecologic…
OncotypeDx helps select women who will benefit from chemotherapy. It identifies women with hormone-responsive breast cancer who have very good outcomes with hormonal therapy…
CARIS Molecular Tumour Profiling is a sophisticated DNA, RNA, Protein and Immune analysis of your tumour. It provides…
Therapy
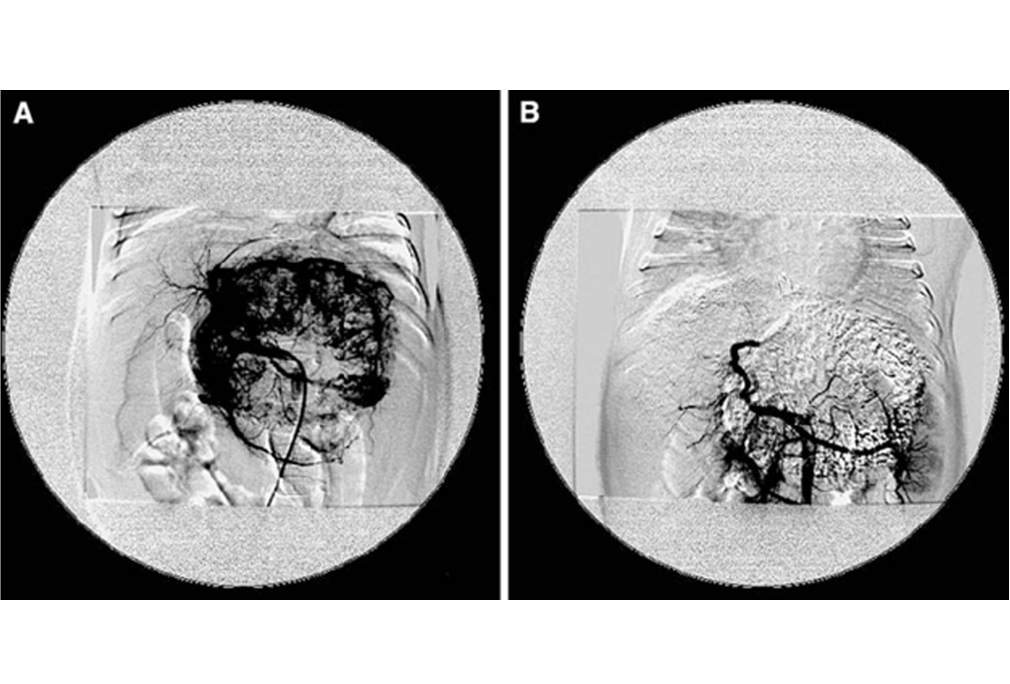
Transarterial chemo-embolization (TACE) involves the administration of cytotoxic chemotherapy directly into the liver tumor via blood vessels. It is useful for delivering…
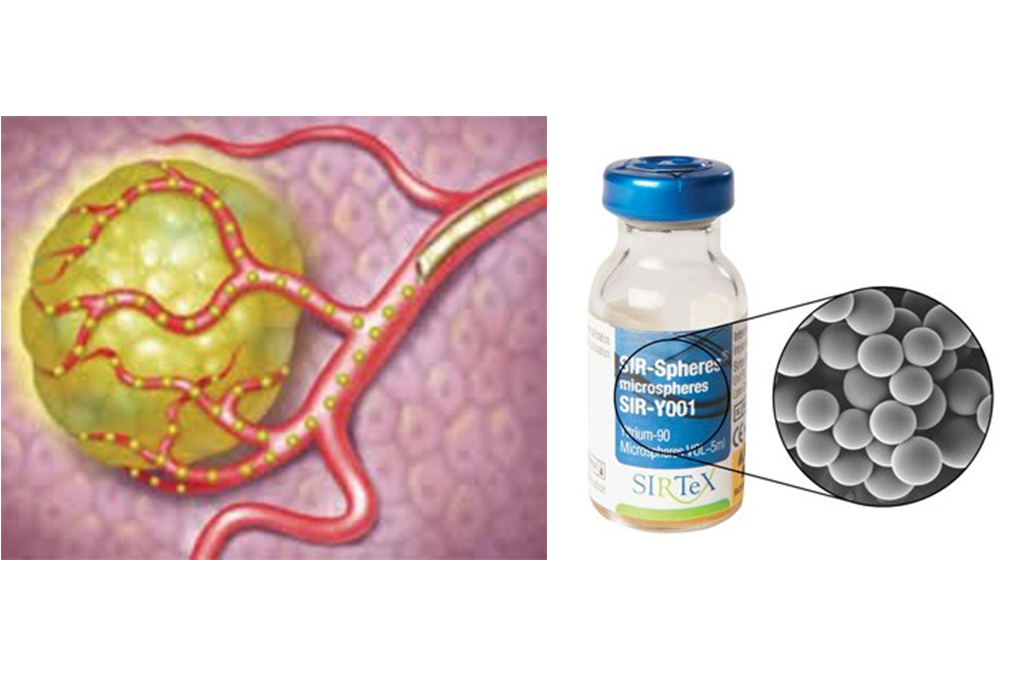
Selective internal radiation therapy (SIRT) or trans-arterial radioembolization (TARE) is a technique where tiny radioactive beads…
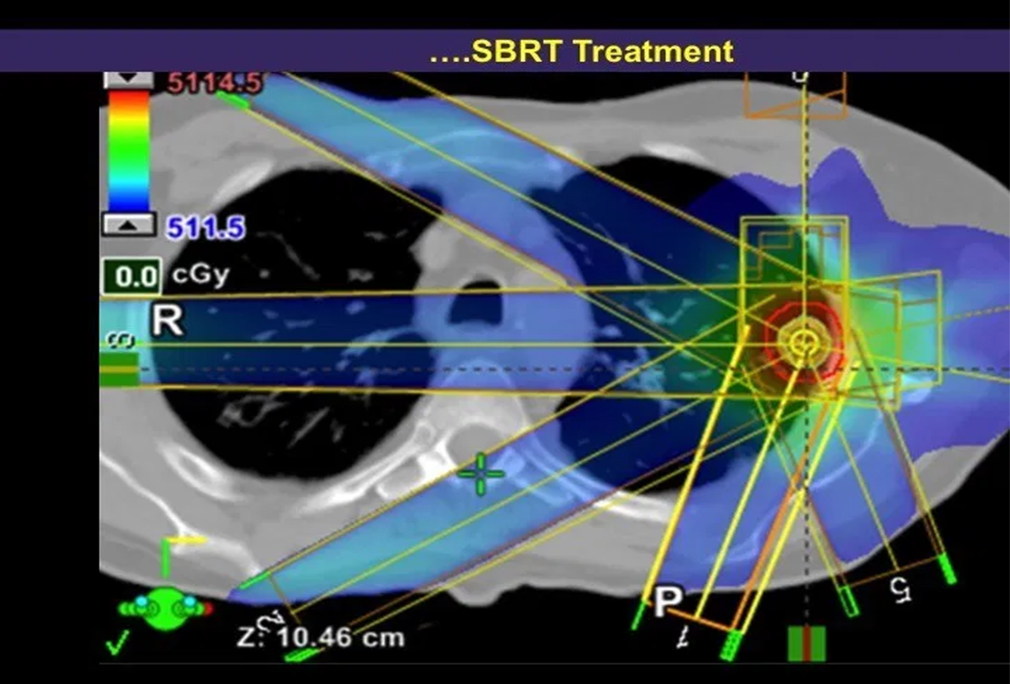
Stereotactic body radiation therapy (SBRT) differs from conventional radiation therapy in allowing the radiation oncologist the ability…
Chemotherapy does not automatically mean you will have hair loss. Scalp cooling with a cold cap device has been shown to be effective in preventing chemotherapy induced hair loss…
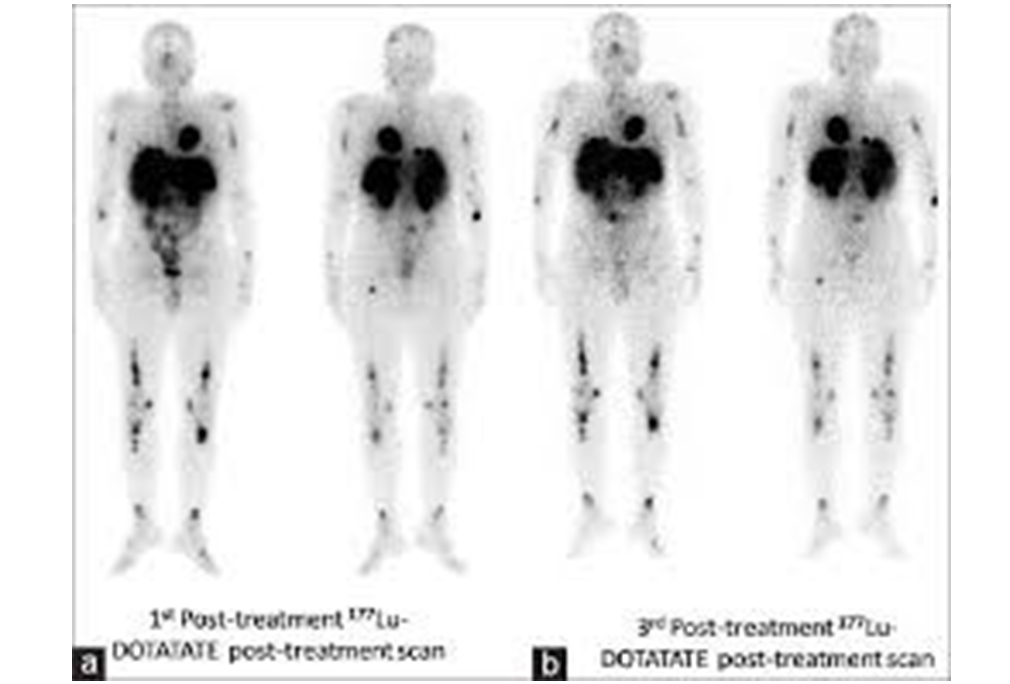
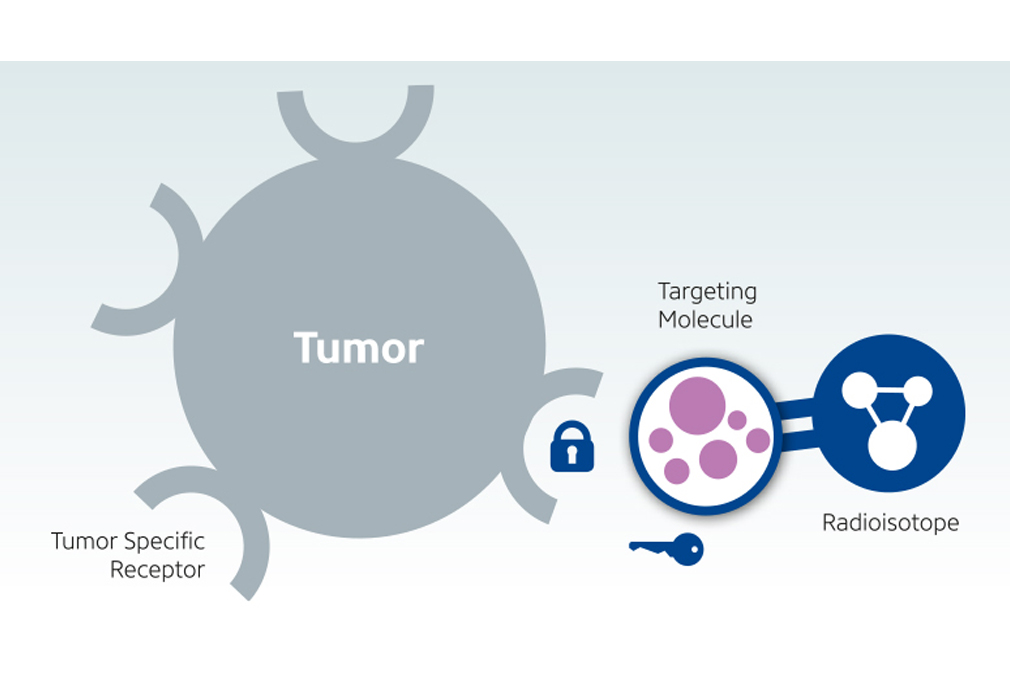
Leutetium-DOTATATE is a form of radioactive targeting therapy that combines DOTATATE, a somatostatin analogue and the radionuclide…
Leutetium-PMSA is a type of targeted therapy that delivers radiation to prostate cancer sparing normal healthy cells. It works by combining…
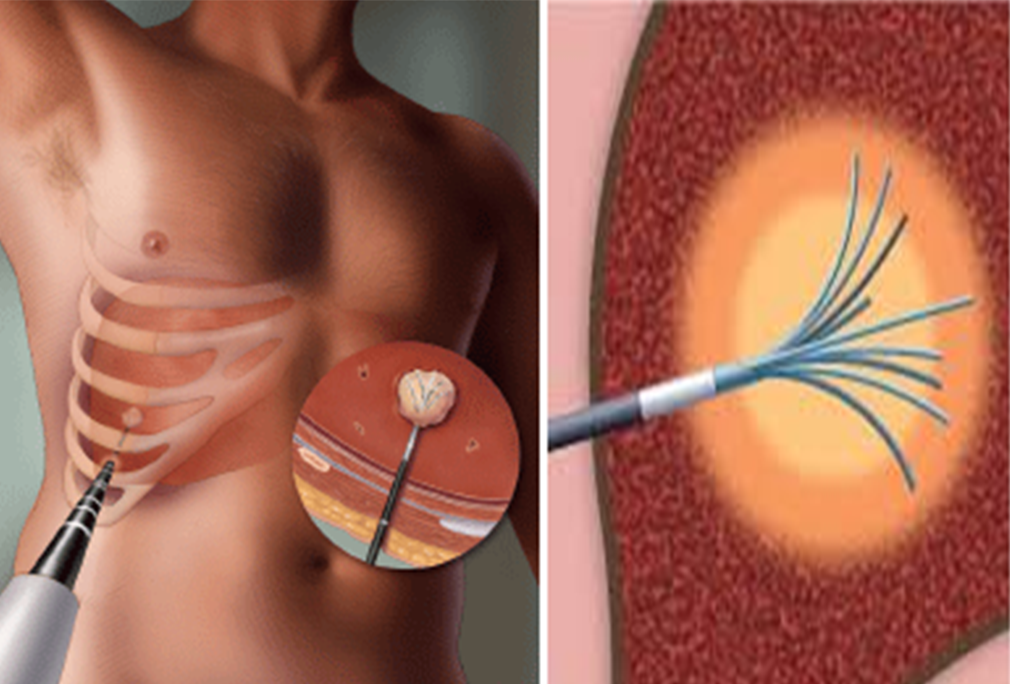
There are many forms of loco-regional therapies used as part of the arsenal against cancer to provide better control of tumors in the liver. Radiofrequency ablation (RFA)and microwave…
Haematopoietic Cell Transplantation (HCT) or Bone Marrow Transplantation: Haematopoietic stem cells are cells that have the ability…